Scientific approach
to help shaping any aspect of your clinical development
Robust recruitment
methodology for
quick patient access
PACE™
High standards in project methodology with
fine-tuned processes
Risked based approach
with preventive QA
Use of state-of-the-art & integrated clinical development technology
Align Our Expertise With Your Needs
We have in-depth experience and expertise to accelerate and perfectly handle the complexity of any biosimilar study. Work together with the pioneers in biosimilars!
Accelsiors is a specialist of clinical trials in special study populations for numerous indications. Our expertise is especially outstanding in pediatric, women’s health, rare diseases and age-group-targeted studies.
Take advantage of our broad experience in the field of innovative drug development from small molecules through complex therapeutic proteins to nanotechnology-formulated development.
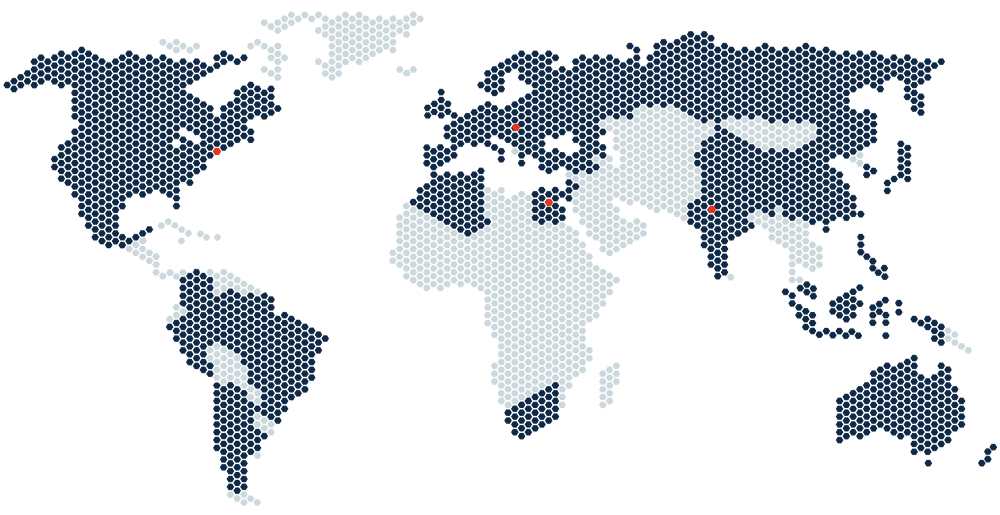
Where Do You Need Us?
Accelsiors operates on a global scale utilizing local expertise to deliver studies within the agreed budget and timelines, meeting the utmost quality expectations. Anywhere you need us…
How Can We Support Your Pipeline?
We are the only resource you need for your clinical trial. We provide end-to-end solution right from inception of study design to site selection to product approval.
Full-Service CRO Solutions
Latest NEWS
News
6th MICROBIOME R&D AND BUSINESS COLLABORATION FORUM PARTICIPATION
Accelsiors is delighted to announce participation at the 6th Microbiome R&D & Business Collaboration Forum, taking place on 20-22 May in Rotterdam, with a dedicated focus on R&D and business collaborations in microbiota research, probiotics, health and disease.
News
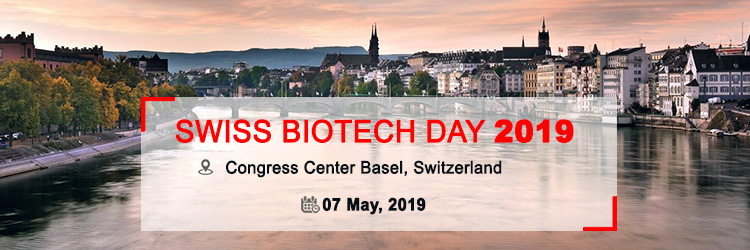
ACCELSIORS TO ATTEND SWISS BIOTECH DAY 2019
Accelsiors CRO is pleased to announce participation at the Swiss Biotech Day 2019 (SBD), taking place on May 7, 2019 in Basel, Switzerland. We are proud to be part of this leading and influential meeting dedicated to demonstrate the power and potential of the Swiss biotech hub.